Tony Moffat, Robert Watt and Sulaf Assi
Department of Pharmaceutical and Biological Chemistry, The School of Pharmacy, University of London, 29–39 Brunswick Square, London WC1N 1AX, UK
Introduction
The counterfeiting of medicines is an increasing world wide problem as a great danger to public health. Counterfeit medicines are imitations of their authentic counterparts so that both physical characterisation and chemical analysis are required to discriminate between them. In this respect, near infrared (NIR) spectroscopy is ideally suited to screening for counterfeit medicines because it is non-destructive, fast, requires no sample preparation, and provides a fingerprint of the physical and chemical composition of a product.1
Pharmaceutical tablets are often a complex mixture of the active pharmaceutical ingredient (API) and excipients (materials added to aid the formulation and manufacture of the product, and its administration). For example, the composition of the core of Viagra tablets is: sildenafil citrate (API), microcrystalline cellulose, calcium hydrogen phosphate (anhydrous), croscarmellose sodium and magnesium stearate. The blue film coat of the tablets comprises: hypromellose, titanium dioxide (E171), lactose, triacetin and indigo carmine aluminium lake (E132). Another example of a more complex matrix is the Anadin brand of ibuprofen tablets 200 mg, which uses 18 different excipients as well as the ibuprofen (API). Pharmaceutical tablets and capsules are manufactured to very tight specifications and, therefore, the chemical and physical “fingerprints” of proprietary products are very constant. A counterfeiter must not only strive to get the physical characteristics of the product the same as the authentic product (colour, markings, shape, size, weight etc.) but also the chemical makeup: same API and excipients at the same concentrations (polymorphic forms, particle sizes etc.). Thus, counterfeit medicines are relatively easy to identify by NIR spectroscopy, which can detect physical as well as chemical differences between products.
Methodology
A simple method for the comparison of authentic and test samples has been used in our laboratory for a number of years. We use a Foss NIRSystems 6500 spectrometer fitted with a Rapid Content Analyser with a lead sulphide detector (1100–2500 nm at 2 nm intervals) with each diffuse reflection spectrum being the mean of 32 scans, which takes about 40 seconds. A critical part of the method is to obtain a batch of the product under test from a trusted source to act as the authentic product for comparison purposes. If the authentic product is a tablet, ten intact tablets from this batch are measured once on each side and the mean standard normal variate (SNV), second derivative (D2) spectrum is taken from these 20 spectra. If the product is a capsule, the contents of 10 capsules are emptied into a Waters Corporation 4 mL glass vial, measured four times with shaking of the vial between scans and the mean SNV-D2 spectrum taken. The test batch is then measured in the same way as the authentic tablet or capsule contents to give a test mean SNV-D2 spectrum. The spectra of the authentic and test products are then compared using correlation in wavelength space. This is a method that is easy to use and relatively fast. It compares the correlation coefficient (r) of the mean test spectrum to the authentic spectrum. A value of r close to 1 means that the test and authentic products are strongly similar, whereas a low r value indicates dissimilarity.

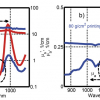
Figure 1 shows the practically superimposable SNV-D2 NIR mean spectra of three batches of authentic Panadol® Actifast Tablets: one batch bought in the UK and two other batches bought in Lebanon. The two Lebanese batches have correlation coefficients of 0.993 and 0.996 compared to the UK batch. This is typical of the batch to batch variation of authentic samples, which nearly always have r values of >0.995. In contrast, Figure 2 clearly shows that the SNV-D2 NIR spectra of the contents of authentic and counterfeit Reductil Capsules 15 mg are very different (r = 0.504). Examination of the peak positions of the differences between the spectra can assist in identifying the raw materials used by the counterfeiters if intelligence information is required. Our experience has shown that the spectra of counterfeit preparations very seldom have a correlation coefficient of >0.95 compared to the spectrum of the authentic product, and this can be used as a threshold with which to identify counterfeit preparations. Thus, test samples are generally reported as authenticated if the correlation coefficient is ≥0.95.

If the value is <0.95, the spectrum is examined for obvious reasons for a failure. Principal components analysis (PCA) can be used to give added confidence in the results, although it is necessary to have many batches of the product to get the best from the use of this chemometric technique. Figure 3 shows the PCA scores plot of the SNV-D2 NIR spectra of Plavix® tablets 75 mg recorded from five authentic batches and two counterfeit products which were labelled as the same batch but obtained from different sources (one from France and one from Egypt). All the authentic batches are relatively close together and clearly discriminated from the two counterfeit batches. If after examining the spectra in detail, comparing by correlation in wavelength space and PCA, there are differences between the authentic and test spectra, we would report the samples as failing the comparison. In this case, either the batch would be examined further by other techniques and/or not passed down the supply chain. PCA can also be used to identify the manufacture of a generic product and sometimes even the site of manufacture. It is also possible to specifically identify the differences in the content of some excipients to extract intelligence information from the spectra, for example, when a different excipient has been used.

We have been working for an international wholesaler for a number of years who wanted to have samples of batches of potential purchases authenticated before they were bought. Of 104 samples submitted for analysis, eight were reported as having failed.
Potential spectral problems
The presence of moisture can be a help or a hindrance. Pharmaceutical products are manufactured under strict conditions of Good Manufacturing Practice (GMP) and the control of the humidity during manufacture is one such controlled condition. Thus, if a tablet product is packed in moisture-proof blister packaging, and the NIR spectrum run immediately the tablet is taken out of the blister, the moisture detected should represent that when it was manufactured. Thus, a higher concentration of water than usual may be helpful to identify counterfeits as they are not normally produced under strictly controlled GMP conditions. If there is any doubt about the storage of the tablets, or if they were not in blister packaging to begin with, it is best to remove the water absorption regions from the mean SNV-D2 spectra (1476–1516 nm and 1918–2089 nm) before a comparison is made.
Because tablets are of different sizes, shapes, colours, embossing etc. it is important to take a sufficient number of spectra of different tablets in a batch so that the resulting mean SNV-D2 spectrum is truly representative of the batch. Taking 20 spectra (10 from each side of the tablet) is a good balance between accuracy and speed of analysis. The SNV and D2 mathematical pre-treatments used before correlation in wavelength space for identification help enormously to remove the multiplicative combinations of the problems of particle size, scatter etc. caused by the different orientations and sides of the tablets in the NIR spectrometer.
The film coating on tablets does not seem to have much effect on the spectra of tablets as it is very thin (3–4 µm). Thus the cores of tablets and their film coated counterparts have very similar spectra. Similarly, blister packaging often has a low absorbance so that the NIR spectra of a tablet in its blister packaging and on its own are very similar. However, this only applies to colourless transparent blisters.2 Opaque white and coloured blisters, and those made of aluminium, do not allow such similarities.
The future
Regulatory agencies around the world are beginning to use NIR spectroscopy to screen for counterfeit medicines, either entering their country or for surveillance operations. The use of portable spectrometers is assisting this process greatly so that analysers can be taken to airports, ports, community pharmacies etc.3 It is most likely that the sale of portable instruments will soon outstrip the sale of laboratory-based instruments and the use of mobile laboratories containing NIR spectrometers will become more widespread as they have in China.1 However, the transferability of spectral libraries from one type of instrument to another will probably still present problems in the years to come. NIR imaging will also become more popular as the instruments are now becoming much lower cost and faster and can be used to identify and quantify individual components in a tablet. Finally, although much work is still required to produce robust methods to quantify the APIs in intact tablets and capsules, there is greater regulatory acceptance of NIR methods in pharmaceutical analysis and process analytical technology.
References
- A.C. Moffat, S. Assi and R.A. Watt, “Identifying counterfeit medicines using near infrared spectroscopy”, J. Near Infrared Spectrosc. 18, 1–15 (2010).
- S. Assi, R.A. Watt and A.C. Moffat, “Identification of tablets through their blister packaging by near-infrared spectroscopy”, J. Pharm. Pharmacol. 61 (Supplement), A31 (2009).
- A.J. O’Neil, R.D. Jee, G. Lee, A. Charvill and A.C. Moffat, “Use of a portable near infrared spectrometer for the authentication of tablets and the detection of counterfeit versions”, J. Near Infrared Spectrosc. 16, 327–333 (2008).