Antony N. Daviesa,b and Robert J. Lancashirec
aStrategic Research Group – Measurement and Analytical Science, Akzo Nobel Chemicals B.V., Deventer, the Netherlands
bSERC, Sustainable Environment Research Centre, Faculty of Computing, Engineering and Science, University of South Wales, UK
cThe Department of Chemistry, The University of the West Indies, Mona Campus, Jamaica
Background
IUPAC Recommendations in the field of nuclear magnetic resonance (NMR) spectroscopy fall into two main categories. First, there are the international data standards related to NMR in the series of JCAMP-DX data formats1–10 and, second, the Recommendations on nomenclature and presentation of NMR data such as those by Robin Harris et al. in 2001 and 2008 and earlier.11–14
These Recommendations deal with standardising practises across a whole field which often become such common usage that practitioners can forget or not even realise why they should carry out a very important task in a particular way. The 2001 Recommendations, for example, standardised the use of the reporting of NMR chemical shifts of all nuclei relative to the 1H resonance of tetramethylsilane (TMS) in dilute (<1%) solution in chloroform. They redefine The Chemical Shift d to better avoid ambiguities in previous definitions and deal with some of the practicalities of using a unified scale: a critical pre-requisite for generating reference databases. They also published very useful tables of the various spin ½, quadrupolar and lanthanide/actinide elements with reference compounds drawn for various literature sources. The focus was very much on solution-state NMR with only a short section on magic angle spinning and solid-state NMR spectroscopy.
In 2008, recent work on the effects of temperature on the TMS signal and more importantly different solvents were addressed and new recommendations published. These included a reference table of the changes in the 1H chemical shift in TMS with various common solvents. This table quoted figures mostly to two decimal places and sometimes only one significant figure. Reporting and referencing of chemical shifts in solids based on high-resolution MAS experiments were dealt with in much more detail.

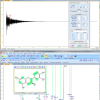
Figure 1. Free induction decay (FID) and 1H NMR spectrum of strychnine, processed using ACD/Labs free software.15
The December 2013 ACS guidelines for publishing spectra16 include:
- Field strength should be noted for each spectrum, not as a comment in the general experimental section.
- The largest peak in the 1H NMR spectrum should normally arise from the compound, not the solvent.
- All peaks in the 1H NMR spectrum should be integrated.
- Chemical shift values should be included.
- The solvent peak should be clearly labelled on the spectrum.
- All peaks should be visible on the spectrum. Insets are encouraged to show expanded regions.
- At minimum, the spectral window should be –1 ppm to 9 ppm for 1H NMR and –10 ppm to 180 ppm for 13C NMR.
Fortunately for us, the vendors do not stand still and with much higher field strengths becoming available to us (e.g. Reference 17) the precision with which we are able to measure chemical shifts has somewhat outgrown that reported in the existing suite of IUPAC Recommendations.
NMR data file converters
In general, data collected from spectrometers are in the form of free induction decays (FIDs) and if a spectrum is stored at all it is in addition to the FID and not as the sole piece of information. The design of the experimental parameters to collect the data and the processing software to display a spectrum are complex and can vary depending on the instrument manufacturer. Storing a spectrum can therefore be useful for archiving and data exchange between sites with different equipment.
File converters have been created by various vendors15,18 (for example, see Figure 1) to deal with the lack of up-to-date, simple, well-documented file exchange formats.19–21 In addition, some spectroscopy software packages have the ability to read a variety of native formats.
NMR data transfer standards
There is currently interest from IUPAC in updating the JCAMP-DX NMR data standards. This is especially relevant for the multi-dimensional NMR standard which was implemented essentially from the distributed final draft when the responsible subcommittee moved to supporting the AnIML initiative at ASTM.
The most recent standard for 1-D NMR is the JCAMP-DX version 5.01 recommendation of 1999.6 This was released with changes needed to cope with Y2K issues and for conformity with good laboratory practices (GLP), amongst other things.
Volunteers sought!
The draft JCAMP-DX version 6 that dealt with multi-dimensional NMR was released for comment but never finalised. According to the AniML team, there is still no timeframe for development of protocols for nD NMR. In the meantime the decision to complete and publish the version 6 standard seems appropriate, but this would require updating with input from both instrument companies and researchers with experience in handling nD NMR spectra.
Clearly, volunteers with experience in handling nD NMR data and willing to help update the draft for publication will need to be recruited. If you think you can contribute, please contact the authors.
References
- R.S. McDonald and P.A. Wilks Jr, “JCAMP-DX: a standard form for exchange of infrared spectra in computer readable form”, Appl. Spectrosc. 42, 151 (1988). doi: http://dx.doi.org/10.1366/0003702884428734
- J.G. Grasselli, “JCAMP-DX, a standard format for exchange of infrared spectra in computer readable form (Recommendations 1991)”, Pure Appl. Chem. 63, 1781 (1991). doi: http://dx.doi.org/10.1351/pac199163121781
- J. Gasteiger, B.M.P. Hendriks, P. Hoever, C. Jochum and H. Somberg, “JCAMP-CS: a standard exchange format for chemical structure information in computer-readable form”, Appl. Spectrosc. 45, 4 (1991). doi: http://dx.doi.org/10.1366/0003702914337894
- A.N. Davies and P. Lampen, “JCAMP-DX for NMR”, Appl. Spectrosc. 47, 1093 (1993). doi: http://dx.doi.org/10.1366/0003702934067874
- P. Lampen, H. Hillig, A.N. Davies and M. Linscheid, “JCAMP-DX for mass spectrometry”, Appl. Spectrosc. 48, 1545 (1994). doi: http://dx.doi.org/10.1366/0003702944027840
- P. Lampen, J. Lambert, R.J. Lancashire, R.S. McDonald, P. McIntyre, D.N. Rutledge, T. Frohlich and A.N. Davies, “An extension to the JCAMP-DX standard file format, JCAMP-DX V.5.01”, Pure Appl. Chem. 71, 1549–1556 (1999). doi: http://dx.doi.org/10.1351/pac199971081549
- A.N. Davies, J. Lambert, R.J. Lancashire, P. Lampen, W. Conover, M. Frey, M. Grzonka, E. Williams and D. Meinhart, “Guidelines for the representation of pulse sequences for solution-state nuclear magnetic resonance spectrometry (IUPAC Recommendations 2001)”, Pure Appl. Chem. 73, 1749 (2001). doi: http://dx.doi.org/10.1351/pac200173111749
- J.I. Baumbach, A.N. Davies, P. Lampen and H. Schmidt, “JCAMP-DX. A standard format for the exchange of ion mobility spectrometry data (IUPAC Recommendations 2001)”, Pure Appl. Chem. 73, 1765 (2001). doi: http://dx.doi.org/10.1351/pac200173111765
- R. Cammack, Y. Fann, R.J. Lancashire, J.P. Maher, P.S. Mcintyre and R. Morse, “JCAMP-DX for electron magnetic resonance (EMR) (IUPAC Recommendations 2006)”, Pure Appl. Chem. 78, 613 (2006). doi: http://dx.doi.org/10.1351/pac200678030613
- B. Woollett, D. Klose, R. Cammack, R.W. Janes and B.A. Wallace, “JCAMP-DX for circular dichroism spectra and metadata (IUPAC Recommendations 2012)”, Pure Appl. Chem. 84, 2171–2182 (2012). doi: http://dx.doi.org/10.1351/PAC-REC-12-02-03
- R.K. Harris, E.D. Becker, S.M. Cabral de Menezes, R. Goodfellow and P. Granger, “NMR nomenclature. Nuclear spin properties and conventions for chemical shifts (IUPAC Recommendations 2001)”, Pure Appl. Chem. 73, 1795–1818 (2001). doi: http://dx.doi.org/10.1351/pac200173111795
- R.K. Harris, E.D. Becker, S.M. Cabral de Menezes, P. Granger, R.E. Hoffman and K.W. Zilm, “Further conventions for NMR shielding and chemical shifts (IUPAC Recommendations 2008)”, Pure Appl. Chem. 80, 59–84 (2008). doi: http://dx.doi.org/10.1351/pac200880010059
- “Recommendations for the presentation of NMR data for publication in chemical journals”, Pure Appl. Chem. 29, 627 (1972). doi: http://dx.doi.org/10.1351/pac197229040625
- “Presentation of NMR data for publication in chemical journals – B. Conventions relating to spectra from nuclei other than protons”, Pure Appl. Chem. 45, 217 (1976). https://www.iupac.org/publications/pac/pdf/1976/pdf/4503x0217.pdf
- http://www.acdlabs.com/resources/freeware/chemsketch/
- NMR guidelines for ACS journals, http://pubs.acs.org/paragonplus/submission/acs_nmr_guidelines.pdf
- A.N. Davies, “GHz NMR”, Spectrosc. Europe 25(3), 18–19 (2013). http://bit.ly/2dfh5GL
- http://mestrelab.com
- R.J. Nowling, J. Vyas, G. Weatherby, M.W. Fenwick, H.J.C. Ellis and MR. Gryk, “CONNJUR spectrum translator: an open source application for reformatting NMR spectral data”, J. Biomol. NMR 50(1), 83–89 (2011). doi: http://dx.doi.org/10.1007/s10858-011-9497-1
- http://www.pascal-man.com/navigation/faq-java-browser/file-conversion.shtml
- http://www.wenmr.eu/wenmr/support/documentation/nmr-services/formatconverter